What Is Subscript In Science? A Complete Guide
Subscripts are a crucial concept across various scientific fields, but what exactly do they mean and when are they used? In this comprehensive guide, we’ll demystify the definition of subscript and how it applies in different contexts.
If you’re short on time, here’s a quick answer to your question: A subscript in science is a number or symbol written slightly below and to the right of another symbol, number or letter to distinguish it or indicate a specific meaning.
We’ll start by explaining the general definition of subscript and where the term originated. We’ll then dive into specific examples of how subscripts are used in chemistry, physics, mathematics and other scientific areas.
You’ll learn the rules around subscript formatting and placement, and how to read expressions with subscripts correctly. By the end, you’ll have a firm grasp of this important science concept so you can apply it confidently in your own work and studies.
Origin and General Definition of Subscript
In scientific notation and mathematical equations, subscripts play an important role in conveying additional information and providing clarity. The use of subscripts originated from the need to differentiate between different elements or variables within a formula or equation.
By adding a subscript, scientists and mathematicians are able to specify which element or variable they are referring to, thus minimizing confusion and allowing for more accurate communication.
What is a Subscript?
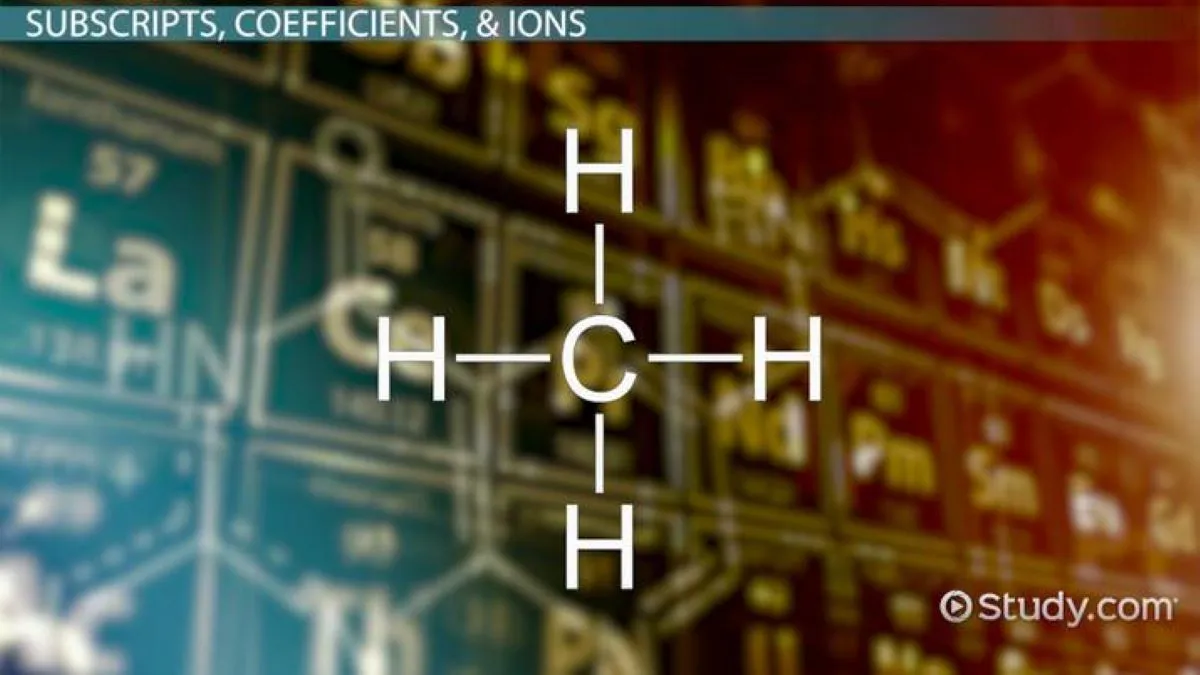
A subscript is a small character or number that is written below the line of text. It is typically used in scientific and mathematical formulas to indicate a specific element, variable, or index. In science, subscripts are commonly used to represent the number of atoms in a chemical formula or the position of an element in a compound.
In mathematics, subscripts are often used to denote the position of a term in a sequence or the value of a variable.
For example, in the chemical formula H2O, the subscript “2” indicates that there are two hydrogen atoms bonded to one oxygen atom. In the equation xn = 2n + 1, the subscript “n” represents the position of the term in the sequence.
Why are Subscripts Used?
The use of subscripts serves several important purposes in science and mathematics. Firstly, they help to differentiate between different elements or variables within a formula or equation. This is especially important in complex equations where multiple variables are involved.
By assigning each variable a unique subscript, scientists and mathematicians can easily identify and manipulate specific elements or variables.
Secondly, subscripts provide valuable information about the structure and composition of chemical compounds. By indicating the number of atoms of each element in a compound, scientists can accurately represent the chemical formula and understand its properties.
This information is crucial for conducting experiments, predicting reactions, and analyzing the behavior of substances.
Common Uses of Subscripts in Science
Subscripts are widely used in various scientific disciplines. Here are some common examples:
- In chemistry, subscripts are used to represent the number of atoms in a chemical formula, the charge of an ion, or the position of an element in a compound.
- In physics, subscripts are used to denote different variables, such as the initial and final states of a system, or the x, y, and z components of a vector.
- In biology, subscripts are used to indicate different isotopes of an element or the number of a specific molecule in a biological process.
Use of Subscripts in Chemistry
Chemical Formulas
In chemistry, subscripts play a crucial role in representing the composition of chemical compounds. A chemical formula is a combination of chemical symbols and subscripts that represent the elements and the number of atoms of each element present in a compound.
For example, the chemical formula for water is H2O, where the subscript 2 indicates that there are two hydrogen atoms bonded to one oxygen atom. Subscripts allow chemists to accurately represent the ratios of elements in a compound and provide essential information about its structure.
Isotopes
Subscripts also come into play when discussing isotopes, which are atoms of the same element but with different numbers of neutrons. Isotopes are represented by the element’s symbol followed by the mass number, which is the sum of the number of protons and neutrons in the nucleus.
For example, carbon has three isotopes: carbon-12, carbon-13, and carbon-14. The subscripts in these isotopes’ symbols indicate their respective mass numbers. Isotopes are crucial in various scientific fields, including radiocarbon dating and medical imaging.
Chemical Reactions
Subscripts are also important in chemical reactions. They indicate the number of atoms or molecules involved in a reaction. In a balanced chemical equation, the subscripts and coefficients are used to show the stoichiometry of the reaction, meaning the ratios of reactants and products.
For example, the balanced equation for the combustion of methane is CH4 + 2O2 → CO2 + 2H2O. The subscripts in this equation show that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
The use of subscripts in chemistry allows scientists to communicate precise information about chemical compounds, isotopes, and reactions. Understanding these subscripts is essential for accurately interpreting chemical formulas and equations.
Subscripts in Physics
Subscripts play a crucial role in physics, as they provide additional information and help clarify certain aspects of scientific notations and formulas. In the realm of physics, subscripts are commonly used to denote different variables or quantities within a given context.
Let’s explore two key areas where subscripts are extensively utilized: vectors and wavefunctions.
Vectors
In physics, vectors are quantities that have both magnitude and direction. They are represented using arrows or bold letters. Subscripts are often used to distinguish between different components of a vector.
For example, in the context of velocity, the subscript “x” may represent the x-component of the vector, and the subscript “y” may represent the y-component. This allows scientists to easily distinguish and work with specific components of a vector, making calculations and analysis more precise and efficient.
Subscripts can also be used to denote different vectors within a given system. For instance, if we have multiple forces acting on an object, we can label them as F1, F2, F3, and so on. This labeling system helps keep track of individual forces and their respective contributions in complex physical systems.
Wavefunctions
Wavefunctions are fundamental in quantum mechanics, describing the behavior of particles in terms of wave-like properties. Subscripts are frequently employed in wavefunctions to indicate the quantum numbers associated with specific energy levels or orbitals.
For example, in the Schrödinger equation, ψn,l,m represents the wavefunction of an electron in an atom, where “n” represents the principal quantum number, “l” represents the azimuthal quantum number, and “m” represents the magnetic quantum number.
Furthermore, subscripts can also be used to differentiate between different particles in a system. In particle physics, the subscript “i” is often used to represent individual particles within a multi-particle system.
This allows for a clear and concise representation of complex particle interactions and calculations.
Understanding the use of subscripts in physics is crucial for accurately representing and analyzing various physical phenomena. By incorporating subscripts, scientists can convey precise information about vectors, wavefunctions, and other important quantities in a concise and organized manner.
So next time you come across a subscript in a physics equation, remember that it carries valuable information that enhances our understanding of the underlying scientific concepts.
Subscripts in Mathematics
Subscripts in Algebra
In algebra, subscripts are used to distinguish between variables or constants with the same letter (x1, x2, x3, etc.). They indicate that the variables are different in some way, but follow the same naming pattern.
Subscripts are common when dealing with sets or sequences of numbers, allowing mathematicians to compactly represent patterns and arrays. For example, an could represent the nth term in a sequence. Subscripts help to organize information and make algebraic expressions less ambiguous.
Subscripts in Calculus
Subscripts have several uses in calculus. They are used to indicate the variable with respect to which we are differentiating or integrating. For example, dy/dx indicates we are finding the derivative of y with respect to x. Subscripts also indicate the lower and upper limits in definite integrals.
For example, ∫ab f(x) dx shows the integral evaluates f(x) from x = a to x = b. Additionally, subscripts can denote which element we are referring to in a set or sequence, like the ith element. This allows summing, product, and limit notation to be written compactly.
Matrices
Subscripts are fundamental to the notation of matrices. The subscript after a matrix indicates its dimensions, like A3×5 is a 3 by 5 matrix. Subscripts after an element indicate its position within the matrix, like a2,3 refers to the element in row 2, column 3.
We can even have double subscripts to represent a matrix of matrices. Subscripts make working with matrices much simpler. Without them, we would need another way to denote the dimensions, rows, columns, and positioning of each element.
Subscript Rules and Formatting
Subscripts are a common feature in science, particularly in formulas and chemical equations. They are used to denote the number of atoms, ions, or molecules in a compound or reaction. Understanding the rules and formatting of subscripts is essential for clear and accurate scientific communication.
1. Placement of Subscripts
Subscripts are typically written slightly below the line of normal text. This ensures that they are clearly distinguished from regular text and are easily identifiable. For example, in the chemical formula H2O, the subscript “2” indicates that there are two hydrogen atoms in a water molecule.
2. Size and Formatting
Subscripts are usually smaller in size compared to the main text. This size difference helps to visually differentiate them and highlights their significance. In most cases, the font size for subscripts is automatically adjusted by word processing software or typesetting systems.
However, it is important to ensure that the subscript remains legible and distinct.
3. Subscript Rules
When using subscripts, it is crucial to follow certain rules to maintain accuracy and clarity. Here are some important rules to keep in mind:
- Subscripts should always be used when denoting the number of atoms or ions in a chemical formula. For example, in CO2, the subscript “2” indicates that there are two oxygen atoms.
- Subscripts are not used to denote the charge of an ion. Instead, superscripts are used for this purpose. For example, in the formula Ca2+, the superscript “+2” indicates that the calcium ion has a charge of 2+.
- Subscripts should be placed immediately after the element symbol or molecule abbreviation. For example, in the formula NH3, the subscript “3” indicates that there are three hydrogen atoms.
- Subscripts should not be used when denoting the number of elements in a compound or the number of molecules in a reaction. Instead, coefficients are used for this purpose. For example, in the balanced equation 2H2O → 2H2 + O2, the coefficient “2” indicates that two molecules of water produce two molecules of hydrogen gas and one molecule of oxygen gas.
By following these rules, scientists can accurately convey the composition and structure of compounds and reactions through the use of subscripts. It is worth noting that proper formatting and adherence to these rules are not only essential for clarity but also for ensuring consistency across scientific literature.
For more information on subscripts and their usage in scientific notation, you can refer to ThoughtCo.com, a reliable source for educational content related to science and mathematics.
Conclusion
In conclusion, subscripts are found across diverse scientific disciplines to distinguish numbers, variables, isotopes, vectors, matrix elements, and more. While the specific meaning changes by context, the general definition remains the same – a subscript is written below and to the right to provide additional information or label specific entities.
Understanding subscripts is key for accurately reading and writing scientific formulas, equations, notations, and texts. We hope this guide provided a comprehensive overview of what subscripts represent in different fields of science, as well as the standard formatting practices to follow.
The next time you encounter a formula or equation with subscripts, you can refer to this article to quickly decipher the meaning and usage of those lowered numbers or symbols. Mastering subscripts will provide a strong foundation for flourishing in your science education and career.